1. http://www.metaboanalyst.ca for statiscal calculation, based on xcms, but user-friendly workflow and UI. ID based on exact mass only.
2. https://xcmsonline.scripps.edu/landing_page.php?pgcontent=mainPage XCMS online version. Need to sign in and upload files. Allows statistical treatment and match with METLIN database.
3. http://mona.fiehnlab.ucdavis.edu/downloads MoNA repository of MS/MS database for spectral similarity search. Downloadable .
4. http://prime.psc.riken.jp/Metabolomics_Software/MS-DIAL/ MS-DIAL allows peak matching for DIA experiment and MS/MS search for data-dependent workflow. Needs to convert data to abf format with https://www.reifycs.com/AbfConverter/.
5. https://msbi.ipb-halle.de/MetFrag/ MetFrag allows in silico fragmentation search
6. https://msbi.ipb-halle.de/MetFusion/ MetFusion allows in silico AND MS/MS search from selected database.
mass_tips
Friday, March 6, 2020
How to setup and run CompassXport
1. Download from Bruker site
2. Click on executable file to install
3. Setup data path (https://www.java.com/en/download/help/path.xml). CompassXport is in C:\Program Files (x86)\Bruker Daltonik\CompassXport
4. lauch Command prompt (https://www.lifewire.com/how-to-open-command-prompt-2618089)
5. At Command prompt, change directory to the directory that contains the data file. (https://www.makeuseof.com/tag/a-beginners-guide-to-the-windows-command-line/)
5a. d:
5b. cd \data\xxx\xx\xx.d
5c. compassxport -mode 2 -a analysis.baf
2. Click on executable file to install
3. Setup data path (https://www.java.com/en/download/help/path.xml). CompassXport is in C:\Program Files (x86)\Bruker Daltonik\CompassXport
4. lauch Command prompt (https://www.lifewire.com/how-to-open-command-prompt-2618089)
5. At Command prompt, change directory to the directory that contains the data file. (https://www.makeuseof.com/tag/a-beginners-guide-to-the-windows-command-line/)
5a. d:
5b. cd \data\xxx\xx\xx.d
5c. compassxport -mode 2 -a analysis.baf
Wednesday, September 4, 2019
Solvent compressibility
Solvent Compressibility
Have you ever noticed pump ripple (baseline noise) that is not caused by a defective check valve ? The ripple might be due to an incorrect solvent compressibility setting.
We normally think of liquids as not being compressible in general. Hydraulic systems take advantage of this physical fact and many innovations have been developed using this concept. However, in high pressure liquid chromatography (HPLC) we routinely subject different liquids to very high pressures which can result in measurable liquid compression. The degree of actual compression varies for each liquid (see table). Though the amount of compression is very small, it is enough to change the flow rate of the system. When multiple solvents are mixed together at different proportions, such as is common when running a gradient, the measured flow rate can vary from the set flow rate during the entire run. This flow rate accuracy issue can be compensated for using the built-in solvent compressibility compensation software which is found in most modern HPLC systems. Many of these systems will allow you to manually enter the actual liquid compressibility values for each solvent (pump channel) used. This can result in better baseline stability and less pump noise. I would like to point out that the small improvement gained in performance is best implemented AFTER other major changes have been addressed first (i.e. such as fully degassing your solvents; filtering samples before injecting; selecting the best signal bandwidth and sampling rate values for your detector and insuring that your pumping system has received regular maintenance).
Note how Water has a compressibility value of ~ 46, but a very common solvent such as Methanol has a value of 120. These two are very different. *Most pumps are pre-set with a compressibility value of '100'. A 50/50 mixture of the two run isocratically might benefit from a manually edited compressibility value of 83 [(46 + 120) = 166 / 2 = 83)]. This is a best guess value as the best compressibility value for a mixture of liquids must be determined through actual experiments. Choose the value which results in the lowest pump pressure ripple and/or noise.
> Bill Letter, 01/16/11.
source: http://www.hplctools.com/Tip_110_HPLC_Solvent_Compressibility_Values.htm
- SOLVENT COMPRESSIBILITY VALUES TABLE
Solvent Compressibility (10-6 per bar) Acetone 126 Acetonitrile 96 Benzene 95 Carbon Tetrachloride 106 Chloroform 100 Cyclohexane 113 Dichloromethane 99 Ethanol 112 Ethyl Acetate 113 Heptane 140 Hexane 158 Isopropanol 100 Methanol 120 Tetrahydrofuran 97 Toluene 90
- The values shown above are approximate and recorded at a temperature of 20C (Handbook of Chemistry and Physics #90). Various grades/purity of solvent may have different compressibility values so please verify the values of your own solvents before use.
Wednesday, July 18, 2018
How to create SPL for ESI system from Bruker
1. Open ESI method. For example xxx.m
2. Open SPL editor and fill in the table
3. Save SPL file under a name. For example xxx_SPL.m
4. Close SPL editor
5. Save the ESI method to xxx_SPL.m
2. Open SPL editor and fill in the table
3. Save SPL file under a name. For example xxx_SPL.m
4. Close SPL editor
5. Save the ESI method to xxx_SPL.m
Monday, August 29, 2016
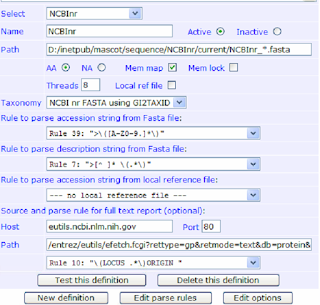
NCBI accession string parse rule for mascot changed sep 2016
According to NCBI, As of Sep 2016, NCBI parse rule for accession string will change to below.
">\([A-Z0-9.]\)"
Description string remain the same
">[^ ]* \(.*\)"
Tuesday, April 7, 2015
How to change and purge pumps
How to change solvent:
1. Start Chromeleon by double-clicking the icon below on the desktop
2. Right-click anywhere on Chromeleon to de-select "Monitor Only" and access HPLC functions. There should be no check-mark in front of "Monitor Only".
Saturday, April 4, 2015
How to submit sample in toxtyper
How to use ToxTyper
1. Launch ToxTyper by clicking on toxtyper icon.
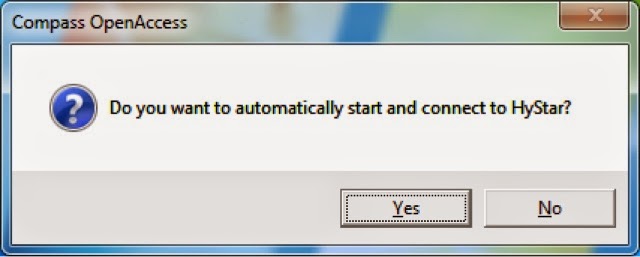
2. Answer "Yes" to "Start HyStar Automatically"
3. System access at Compass OpenAccess
4. Click on Queue Job(s)
5. Log in with user name and password then click "Next>". Now use demo.
6. Supply sample name (ID) and select Method for analysis (doa = drug of abuse, toxtyper = general, toxtyper QC = QC standard for instrument check)
7. Place sample vial on the specified position (R = Red, G = Green, B = Blue tray) , mark position and click Finish
Subscribe to:
Comments (Atom)